Há alguns dias o nosso estagiário, Ícarus, postou alguns tutoriais de bombas caseiras. Nos comentários, um leitor, de nick Merling, postou:
"A próxima pode ser nuclear??? Onde arrumaremos urânio? ... Dear Santa..."
Bom, atendendo ao pedido [isso foi um pedido?], vou postar o diagrama que consegui de uma bomba nuclear.
"ahhh, mas está em inglês D= ". Foda-se. não encontrei em português e nem vou traduzir.
Ps: O Projeto Ensine Rapidamente Algo Inútil, seus produtores, parceiros, contribuidores e comunidade não se responsabilizam por nada. Qualquer coisa, a culpa não foi nossa.
--------------------------------
File courtesy of Outlaw Labs
--------------------------------
=========================================================================
-------------------------------------------------
- Documentation and Diagrams of the Atomic Bomb -
-------------------------------------------------
=========================================================================
______________
/ \
<-} DISCLAIMER {->
\______________/
The information contained in this file is strictly for
academic use alone. Outlaw Labs will bear no responsibility for any use otherwise.
It would be wise to note that the personnel who design and construct
these devices are skilled physicists and are more knowledgeable in these
matters than any layperson can ever hope to be... Should a layperson
attempt to build a device such as this, chances are s/he would probably kill
his/herself not by a nuclear detonation, but rather through radiation exposure. We
here at Outlaw Labs do not recommend using this file beyond the realm of
casual or academic curiosity.
=========================================================================
-----------------------
-+ Table of Contents +-
-----------------------
I. The History of the Atomic Bomb
------------------------------
A). Development (The Manhattan Project)
B). Detonation
1). Hiroshima
2). Nagasaki
3). Byproducts of atomic detonations
4). Blast Zones
II. Nuclear Fission/Nuclear Fusion
------------------------------
A). Fission (A-Bomb) & Fusion (H-Bomb)
B). U-235, U-238 and Plutonium
III. The Mechanism of The Bomb
-------------------------
A). Altimeter
B). Air Pressure Detonator
C). Detonating Head(s)
D). Explosive Charge(s)
E). Neutron Deflector
F). Uranium & Plutonium
G). Lead ShielH). Fuses
IV. The Diagram of The Bomb
-----------------------
A). The Uranium Bomb
B). The Plutonium Bomb
=========================================================================
--------------------------------
File courtesy of Outlaw Labs
--------------------------------
I. The History of the Atomic Bomb
------------------------------
On August 2nd 1939, just before the beginning of World War II,
Albert Einstein wrote to then President Franklin D. Roosevelt. Einstein and
several other scientists told Roosevelt of efforts in Nazi Germany to purify U-235
with which might in turn be used to build an atomic bomb. It was shortly
thereafter that the United States Government began the serious undertaking
known only then as the Manhattan Project. Simply put, the Manhattan Project
was committed to expedient research and production that would produce a
viable atomic bomb.
The most complicated issue to be addressed was the production of ample
amounts of `enriched' uranium to sustain a chain reaction. At the time,
Uranium-235 was very hard to extract. In fact, the ratio of conversion
from Uranium ore to Uranium metal is 500:1. An additional drawback is that
the 1 part of Uranium that is finally refined from the ore consists of over 99%
Uranium-238, which is practically useless for an atomic bomb. To make it
even more difficult, U-235 and U-238 are precisely similar in their chemical
makeup. This proved to be as much of a challenge as separating a
solution of sucrose from a solution of glucose. No ordinary chemical extraction
could separate the two isotopes. Only mechanical methods could effectively
separate U-235 from U-238. Several scientists at Columbia University managed to
solve this dilemma.
A massive enrichment laboratory/plant was constructed at Oak Ridge,
Tennessee. H.C. Urey, along with his associates and colleagues at
Columbia University, devised a system that worked on the principle of gaseous
diffusion. Following this process, Ernest O. Lawrence (inventor of the
Cyclotron) at the University of California in Berkeley implemented a
process involving magnetic separation of the two isotopes.
Following the first two processes, a gas centrifuge was used to
further separate the lighter U-235 from the heavier non-fissionable U-238 by
their mass. Once all of these procedures had been completed, all that needed
to be done was to put to the test the entire concept behind atomic fission.
[For more information on these procedures of refining Uranium, see Section 3.]
Over the course of six years, ranging from 1939 to 1945, more than 2
billion dollars were spent on the Manhattan Project. The formulas for
refining Uranium and putting together a working bomb were created and
seen to their logical ends by some of the greatest minds of our time. Among
these people who unleashed the power of the atomic bomb was J. Robert
Oppenheimer.
Oppenheimer was the major force behind the Manhattan Project. He
literally ran the show and saw to it that all of the great minds working
on this project made their brainstorms work. He oversaw the entire project
from its conception to its completion.
Finally the day came when all at Los Alamos would find out whether
or not The Gadget (code-named as such during its development) was either going
to be the colossal dud of the century or perhaps end the war. It all came down
to a fateful morning of midsummer, 1945.
At 5:29:45 (Mountain War Time) on July 16th, 1945, in a white blaze that
stretched from the basin of the Jemez Mountains in northern New Mexico to the
still-dark skies, The Gadget ushered in the Atomic Age. The light of the
explosion then turned orange as the atomic fireball began shooting
upwards at 360 feet per second, reddening and pulsing as it cooled. The
characteristic mushroom cloud of radioactive vapor materialized at 30,000 feet. Beneath
the cloud, all that remained of the soil at the blast site were fragments of
jade green radioactive glass. ...All of this caused by the heat of the
reaction.
The brilliant light from the detonation pierced the early morning skies
with such intensity that residents from a faraway neighboring community
would swear that the sun came up twice that day. Even more astonishing is that
a blind girl saw the flash 120 miles away.
Upon witnessing the explosion, reactions among the people who
created it were mixed. Isidor Rabi felt that the equilibrium in nature had been
upset -- as if humankind had become a threat to the world it inhabited.
J. Robert Oppenheimer, though ecstatic about the success of the project,
quoted a remembered fragment from Bhagavad Gita. "I am become Death," he
said, "the destroyer of worlds." Ken Bainbridge, the test director, told
Oppenheimer, "Now we're all sons of bitches."
Several participants, shortly after viewing the results, signed
petitions against loosing the monster they had created, but their protests fell on
deaf ears. As it later turned out, the Jornada del Muerto of New Mexico was
not the last site on planet Earth to experience an atomic explosion.
As many know, atomic bombs have been used only twice in warfare.
The first and foremost blast site of the atomic bomb is Hiroshima. A Uranium
bomb (which weighed in at over 4 & 1/2 tons) nicknamed "Little Boy" was
dropped on Hiroshima August 6th, 1945. The Aioi Bridge, one of 81
bridges connecting the seven-branched delta of the Ota River, was the aiming
point of the bomb. Ground Zero was set at 1,980 feet. At 0815 hours, the bomb
was dropped from the Enola Gay. It missed by only 800 feet. At 0816 hours,
in the flash of an instant, 66,000 people were killed and 69,000 people were
injured by a 10 kiloton atomic explosion.
The point of total vaporization from the blast measured one half of
a mile in diameter. Total destruction ranged at one mile in diameter.
Severe blast damage carried as far as two miles in diameter. At two and a half
miles, everything flammable in the area burned. The remaining area of
the blast zone was riddled with serious blazes that stretched out to the
final edge at a little over three miles in diameter. [See diagram below for
blast ranges from the atomic blast.]
On August 9th 1945, Nagasaki fell to the same treatment as
Hiroshima. Only this time, a Plutonium bomb nicknamed "Fat Man" was dropped on the
city. Even though the "Fat Man" missed by over a mile and a half, it still
leveled nearly half the city. Nagasaki's population dropped in one split-second
from 422,000 to 383,000. 39,000 were killed, over 25,000 were injured. That
blast was less than 10 kilotons as well. Estimates from physicists who
have studied each atomic explosion state that the bombs that were used had
utilized only 1/10th of 1 percent of their respective explosive capabilities.
While the mere explosion from an atomic bomb is deadly enough, its
destructive ability doesn't stop there. Atomic fallout creates another
hazard as well. The rain that follows any atomic detonation is laden with
radioactive particles. Many survivors of the Hiroshima and Nagasaki
blasts succumbed to radiation poisoning due to this occurance.
The atomic detonation also has the hidden lethal surprise of
affecting the future generations of those who live through it. Leukemia is among
the greatest of afflictions that are passed on to the offspring of survivors.
While the main purpose behind the atomic bomb is obvious, there are
many by-products that have been brought into consideration in the use of all
weapons atomic. With one small atomic bomb, a massive area's
communications, travel and machinery will grind to a dead halt due to the EMP (Electro-
Magnetic Pulse) that is radiated from a high-altitude atomic detonation.
These high-level detonations are hardly lethal, yet they deliver a
serious enough EMP to scramble any and all things electronic ranging from copper
wires all the way up to a computer's CPU within a 50 mile radius.
At one time, during the early days of The Atomic Age, it was a
popular notion that one day atomic bombs would one day be used in mining
operations and perhaps aid in the construction of another Panama Canal. Needless to
say, it never came about. Instead, the military applications of atomic
destruction increased. Atomic tests off of the Bikini Atoll and several other sites
were common up until the Nuclear Test Ban Treaty was introduced. Photos of
nuclear test sites here in the United States can be obtained through the Freedom
of Information Act.
=========================================================================
---------------------
- Diagram Outline -
---------------------
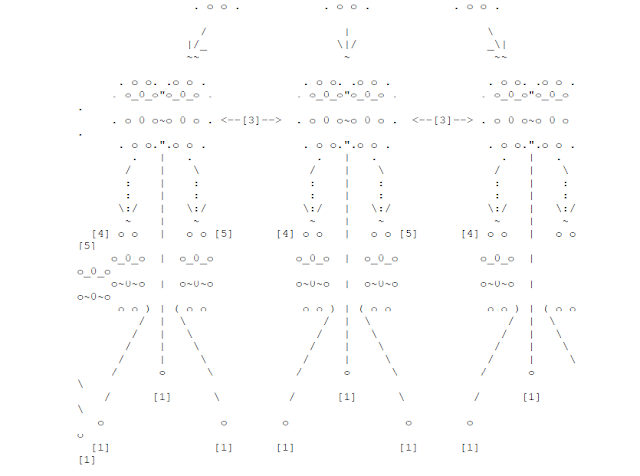
[1] Vaporization Point
------------------
Everything is vaporized by the atomic blast. 98% fatalities.
Overpress=25 psi. Wind velocity=320 mph.
[2] Total Destruction
-----------------
All structures above ground are destroyed. 90% fatalities.
Overpress=17 psi. Wind velocity=290 mph.
[3] Severe Blast Damage
-------------------
Factories and other large-scale building collapse. Severe
damage to highway bridges. Rivers sometimes flow countercurrent.
65% fatalities, 30% injured.
Overpress=9 psi. Wind velocity=260 mph.
[4] Severe Heat Damage
------------------
Everything flammable burns. People in the area suffocate due
to the fact that most available oxygen is consumed by the fires.
50% fatalities, 45% injured.
Overpress=6 psi. Wind velocity=140 mph.
[5] Severe Fire & Wind Damage
-------------------------
Residency structures are severely damaged. People are blown
around. 2nd and 3rd-degree burns suffered by most survivors.
15% dead. 50% injured.
Overpress=3 psi. Wind velocity=98 mph.
=========================================================================
-End of section 1-
--------------------------------
File courtesy of Outlaw Labs
-End of section 1-
--------------------------------
File courtesy of Outlaw Labs
=========================================================================
--------------------------------
II. Nuclear Fission/Nuclear Fusion
------------------------------
There are 2 types of atomic explosions that can be facilitated by U-
235; fission and fusion. Fission, simply put, is a nuclear reaction in which
an atomic nucleus splits into fragments, usually two fragments of comparable
mass, with the evolution of approximately 100 million to several hundred
million volts of energy. This energy is expelled explosively and
violently in the atomic bomb. A fusion reaction is invariably started with a fission
reaction, but unlike the fission reaction, the fusion (Hydrogen) bomb
derives its power from the fusing of nuclei of various hydrogen isotopes in the
formation of helium nuclei. Being that the bomb in this file is strictly
atomic, the other aspects of the Hydrogen Bomb will be set aside for now.
The massive power behind the reaction in an atomic bomb arises from
the forces that hold the atom together. These forces are akin to, but not
quite the same as, magnetism.
Atoms are comprised of three sub-atomic particles. Protons and neutrons
cluster together to form the nucleus (central mass) of the atom while the
electrons orbit the nucleus much like planets around a sun. It is these
particles that determine the stability of the atom.
Most natural elements have very stable atoms which are impossible to
split except by bombardment by particle accelerators. For all practical
purposes, the one true element whose atoms can be split comparatively
easily is the metal Uranium. Uranium's atoms are unusually large, henceforth,
it is hard for them to hold together firmly. This makes Uranium-235 an
exceptional candidate for nuclear fission.
Uranium is a heavy metal, heavier than gold, and not only does it
have the largest atoms of any natural element, the atoms that comprise Uranium
have far more neutrons than protons. This does not enhance their capacity to
split, but it does have an important bearing on their capacity to
facilitate an explosion.
There are two isotopes of Uranium. Natural Uranium consists mostly
of isotope U-238, which has 92 protons and 146 neutrons (92+146=238). Mixed
with this isotope, one will find a 0.6% accumulation of U-235, which has only
143 neutrons. This isotope, unlike U-238, has atoms that can be split, thus
it is termed "fissionable" and useful in making atomic bombs. Being that U-238
is neutron-heavy, it reflects neutrons, rather than absorbing them like its
brother isotope, U-235. (U-238 serves no function in an atomic reaction,
but its properties provide an excellent shield for the U-235 in a constructed
bomb as a neutron reflector. This helps prevent an accidental chain reaction
between the larger U-235 mass and its `bullet' counterpart within the
bomb.
Also note that while U-238 cannot facilitate a chain-reaction, it can be
neutron-saturated to produce Plutonium (Pu-239). Plutonium is
fissionable and can be used in place of Uranium-235 {albeit, with a different model of
detonator} in an atomic bomb. [See Sections 3 & 4 of this file.])
Both isotopes of Uranium are naturally radioactive. Their bulky
atoms disintegrate over a period of time. Given enough time, (over 100,000
years or more) Uranium will eventually lose so many particles that it will turn
into the metal lead. However, this process can be accelerated. This process
is known as the chain reaction. Instead of disintegrating slowly, the atoms
are forcibly split by neutrons forcing their way into the nucleus. A U-235
atom is so unstable that a blow from a single neutron is enough to split it
and henceforth bring on a chain reaction. This can happen even when a
critical mass is present. When this chain reaction occurs, the Uranium atom
splits into two smaller atoms of different elements, such as Barium and Krypton.
When a U-235 atom splits, it gives off energy in the form of heat and
Gamma radiation, which is the most powerful form of radioactivity and the
most lethal. When this reaction occurs, the split atom will also give off two
or three of its `spare' neutrons, which are not needed to make either Barium
or Krypton. These spare neutrons fly out with sufficient force to split
other atoms they come in contact with. [See chart below] In theory, it is
necessary to split only one U-235 atom, and the neutrons from this will
split other atoms, which will split more...so on and so forth. This
progression does not take place arithmetically, but geometrically. All of this will
happen within a millionth of a second.
The minimum amount to start a chain reaction as described above is
known as SuperCritical Mass. The actual mass needed to facilitate this chain
reaction depends upon the purity of the material, but for pure U-235, it
is 110 pounds (50 kilograms), but no Uranium is never quite pure, so in
reality more will be needed.
Uranium is not the only material used for making atomic bombs.
Another material is the element Plutonium, in its isotope Pu-239. Plutonium is
not found naturally (except in minute traces) and is always made from
Uranium. The only way to produce Plutonium from Uranium is to process U-238
through a nuclear reactor. After a period of time, the intense radioactivity
causes the metal to pick up extra particles, so that more and more of its atoms turn
into Plutonium.
Plutonium will not start a fast chain reaction by itself, but this
difficulty is overcome by having a neutron source, a highly radioactive
material that gives off neutrons faster than the Plutonium itself. In
certain types of bombs, a mixture of the elements Beryllium and Polonium is used
to bring about this reaction. Only a small piece is needed. The material
is not fissionable in and of itself, but merely acts as a catalyst to the
greater reaction.
- Diagram Outline -
---------------------
[1] - Incoming Neutron
[2] - Uranium-235
[3] - Uranium-236
[4] - Barium Atom
[5] - Krypton Atom
=========================================================================
-End of section 2-
-Diagrams & Documentation of the Atomic Bomb-
--------------------------------
File courtesy of Outlaw Labs
-------------------------
Altimeter
---------
An ordinary aircraft altimeter uses a type of Aneroid Barometer
which measures the changes in air pressure at different heights. However,
changes in air pressure due to the weather can adversely affect the altimeter's
readings. It is far more favorable to use a radar (or radio) altimeter
for enhanced accuracy when the bomb reaches Ground Zero.
While Frequency Modulated-Continuous Wave (FM CW) is more
complicated, the accuracy of it far surpasses any other type of altimeter. Like
simple pulse systems, signals are emitted from a radar aerial (the bomb),
bounced off the ground and received back at the bomb's altimeter. This pulse system
applies to the more advanced altimeter system, only the signal is
continuous and centered around a high frequency such as 4200 MHz. This signal is
arranged to steadily increase at 200 MHz per interval before dropping
back to its original frequency.
As the descent of the bomb begins, the altimeter transmitter will send
out a pulse starting at 4200 MHz. By the time that pulse has returned, the
altimeter transmitter will be emitting a higher frequency. The
difference depends on how long the pulse has taken to do the return journey. When
these two frequencies are mixed electronically, a new frequency (the difference
between the two) emerges. The value of this new frequency is measured by
the built-in microchips. This value is directly proportional to the distance
travelled by the original pulse, so it can be used to give the actual
height.
In practice, a typical FM CW radar today would sweep 120 times per
second. Its range would be up to 10,000 feet (3000 m) over land and
20,000 feet (6000 m) over sea, since sound reflections from water surfaces are
clearer.
The accuracy of these altimeters is within 5 feet (1.5 m) for the higher
ranges. Being that the ideal airburst for the atomic bomb is usually set
for 1,980 feet, this error factor is not of enormous concern.
The high cost of these radar-type altimeters has prevented their use
in commercial applications, but the decreasing cost of electronic components
should make them competitive with barometric types before too long.
Air Pressure Detonator
----------------------
The air pressure detonator can be a very complex mechanism, but for
all practical purposes, a simpler model can be used. At high altitudes, the
air is of lesser pressure. As the altitude drops, the air pressure
increases. A simple piece of very thin magnetized metal can be used as an air pressure
detonator. All that is needed is for the strip of metal to have a bubble
of extremely thin metal forged in the center and have it placed directly
underneath the electrical contact which will trigger the conventional
explosive detonation. Before setting the strip in place, push the bubble in
so that it will be inverted.
Once the air pressure has achieved the desired level, the magnetic
bubble will snap back into its original position and strike the contact, thus
completing the circuit and setting off the explosive(s).
Detonating Head
---------------
The detonating head (or heads, depending on whether a Uranium or
Plutonium bomb is being used as a model) that is seated in the
conventional explosive charge(s) is similar to the standard-issue blasting cap. It
merely serves as a catalyst to bring about a greater explosion. Calibration of
this device is essential. Too small of a detonating head will only cause a
colossal dud that will be doubly dangerous since someone's got to disarm
and re-fit the bomb with another detonating head. (an added measure of
discomfort comes from the knowledge that the conventional explosive may have
detonated with insufficient force to weld the radioactive metals. This will cause
a supercritical mass that could go off at any time.) The detonating head
will receive an electric charge from the either the air pressure detonator or
the radar altimeter's coordinating detonator, depending on what type of
system is used. The Du Pont company makes rather excellent blasting caps that can
be easily modified to suit the required specifications.
Conventional Explosive Charge(s)
--------------------------------
This explosive is used to introduce (and weld) the lesser amount of
Uranium to the greater amount within the bomb's housing. [The amount of
pressure needed to bring this about is unknown and possibly classified by
the United States Government for reasons of National Security]
Plastic explosives work best in this situation since they can be
manipulated to enable both a Uranium bomb and a Plutonium bomb to
detonate.
One very good explosive is Urea Nitrate. The directions on how to make
Urea
Nitrate are as follows:
- Ingredients -
---------------
[1] 1 cup concentrated solution of uric acid (C5 H4 N4 O3)
[2] 1/3 cup of nitric acid
[3] 4 heat-resistant glass containers
[4] 4 filters (coffee filters will do)
Filter the concentrated solution of uric acid through a filter to
remove
impurities. Slowly add 1/3 cup of nitric acid to the solution and let
the mixture stand for 1 hour. Filter again as before. This time the Urea
Nitrate crystals will collect on the filter. Wash the crystals by pouring water
over them while they are in the filter. Remove the crystals from the filter
and allow 16 hours for them to dry. This explosive will need a blasting cap
to detonate.
It may be necessary to make a quantity larger than the
aforementioned list calls for to bring about an explosion great enough to cause the
Uranium (or Plutonium) sections to weld together on impact.
Neutron Deflector
-----------------
The neutron deflector is comprised solely of Uranium-238. Not only
is U-238 non-fissionable, it also has the unique ability to reflect neutrons
back to their source.
The U-238 neutron deflector can serve 2 purposes. In a Uranium
bomb, the neutron deflector serves as a safeguard to keep an accidental
supercritical mass from occurring by bouncing the stray neutrons from the `bullet'
counterpart of the Uranium mass away from the greater mass below it (and
viceversa).
The neutron deflector in a Plutonium bomb actually helps the
wedges of Plutonium retain their neutrons by `reflecting' the stray particles
back into the center of the assembly. [See diagram in Section 4 of this
file.]
Uranium & Plutonium
-------------------
Uranium-235 is very difficult to extract. In fact, for every 25,000
tons of Uranium ore that is mined from the earth, only 50 tons of Uranium
metal can be refined from that, and 99.3% of that metal is U-238 which is too
stable to be used as an active agent in an atomic detonation. To make matters even
more complicated, no ordinary chemical extraction can separate the two
isotopes since both U-235 and U-238 possess precisely identical chemical
characteristics. The only methods that can effectively separate U-235 from
U-238 are mechanical methods.
U-235 is slightly, but only slightly, lighter than its counterpart,
U-238. A system of gaseous diffusion is used to begin the separating
process between the two isotopes. In this system, Uranium is combined with
fluorine to form Uranium Hexafluoride gas. This mixture is then propelled by lowpressure
pumps through a series of extremely fine porous barriers.
Because
the U-235 atoms are lighter and thus propelled faster than the U-238
atoms, they could penetrate the barriers more rapidly. As a result, the
U-235's concentration became successively greater as it passed through
each barrier. After passing through several thousand barriers, the Uranium
Hexafluoride contains a relatively high concentration of U-235 -- 2% pure
Uranium in the case of reactor fuel, and if pushed further could
(theoretically) yield up to 95% pure Uranium for use in an atomic bomb.
Once the process of gaseous diffusion is finished, the Uranium must
be refined once again. Magnetic separation of the extract from the previous
enriching process is then implemented to further refine the Uranium.
This involves electrically charging Uranium Tetrachloride gas and directing it
past a weak electromagnet. Since the lighter U-235 particles in the gas
stream are less affected by the magnetic pull, they can be gradually separated from
the flow.
Following the first two procedures, a third enrichment process is
then applied to the extract from the second process. In this procedure, a gas
centrifuge is brought into action to further separate the lighter U-235
from its heavier counter-isotope. Centrifugal force separates the two
isotopes of Uranium by their mass. Once all of these procedures have been completed,
all that need be done is to place the properly molded components of Uranium-
235 inside a warhead that will facilitate an atomic detonation.
Supercritical mass for Uranium-235 is defined as 110 lbs (50 kgs) of
pure Uranium.
Depending on the refining process(es) used when purifying the U-235
for use, along with the design of the warhead mechanism and the altitude at
which it detonates, the explosive force of the A-bomb can range anywhere from 1
kiloton (which equals 1,000 tons of TNT) to 20 megatons (which equals 20
million tons of TNT -- which, by the way, is the smallest strategic
nuclear warhead we possess today. {Point in fact -- One Trident Nuclear
Submarine carries as much destructive power as 25 World War II's}).
While Uranium is an ideally fissionable material, it is not the only
one.
Plutonium can be used in an atomic bomb as well. By leaving U-238 inside
an atomic reactor for an extended period of time, the U-238 picks up extra
particles (neutrons especially) and gradually is transformed into the
element Plutonium.
Plutonium is fissionable, but not as easily fissionable as Uranium.
While Uranium can be detonated by a simple 2-part gun-type device,
Plutonium must be detonated by a more complex 32-part implosion chamber along with
a stronger conventional explosive, a greater striking velocity and a
simultaneous triggering mechanism for the conventional explosive packs.
Along with all of these requirements comes the additional task of introducing a
fine mixture of Beryllium and Polonium to this metal while all of these
actions are occurring.
Supercritical mass for Plutonium is defined as 35.2 lbs (16 kgs).
This amount needed for a supercritical mass can be reduced to a smaller
quantity of 22 lbs (10 kgs) by surrounding the Plutonium with a U-238 casing.
To illustrate the vast difference between a Uranium gun-type
detonator and a Plutonium implosion detonator, here is a quick rundown.
=========================================================================
[1] Uranium Detonator
-----------------
Comprised of 2 parts. Larger mass is spherical and
concave.
Smaller mass is precisely the size and shape of the
`missing' section of the larger mass. Upon detonation of
conventional explosive, the smaller mass is violently injected and
welded to the larger mass. Supercritical mass is reached, chain
reaction follows in one millionth of a second.
[2] Plutonium Detonator
-------------------
Comprised of 32 individual 45-degree pie-shaped sections of
Plutonium surrounding a Beryllium/Polonium mixture. These
32 sections together form a sphere. All of these sections
must have the precisely equal mass (and shape) of the others.
The shape of the detonator resembles a soccerball. Upon
detonation of conventional explosives, all 32 sections must merge with
the B/P mixture within 1 ten-millionths of a second.
=========================================================================
Lead Shield
-----------
The lead shield's only purpose is to prevent the inherent
radioactivity of the bomb's payload from interfering with the other mechanisms of the
bomb.
The neutron flux of the bomb's payload is strong enough to short circuit
the internal circuitry and cause an accidental or premature detonation.
Fuses
-----
The fuses are implemented as another safeguard to prevent an
accidental detonation of both the conventional explosives and the nuclear payload.
These fuses are set near the surface of the `nose' of the bomb so that they can
be installed easily when the bomb is ready to be launched. The fuses should
be installed only shortly before the bomb is launched. To affix them before
it is time could result in an accident of catastrophic proportions.
=========================================================================
-End of section 3-
-Documentation & Diagrams of the Atomic Bomb-
--------------------------------
File courtesy of Outlaw Labs
--------------------------------
=========================================================================
- Diagram Outline -
---------------------
[1] - Tail Cone
[2] - Stabilizing Tail Fins
[3] - Air Pressure Detonator
[4] - Air Inlet Tube(s)
[5] - Altimeter/Pressure Sensors
[6] - Lead Shield Container
[7] - Detonating Head
[8] - Conventional Explosive Charge
[9] - Packing
[10] - Uranium (U-235) [Plutonium (See other diagram)]
[11] - Neutron Deflector (U-238)
[12] - Telemetry Monitoring Probes
[13] - Receptacle for U-235 upon detonation
to facilitate supercritical mass.
[14] - Fuses (inserted to arm bomb)
---------------------
[1] - Tail Cone
[2] - Stabilizing Tail Fins
[3] - Air Pressure Detonator
[4] - Air Inlet Tube(s)
[5] - Altimeter/Pressure Sensors
[6] - Electronic Conduits & Fusing Circuits
[7] - Lead Shield Container
[8] - Neutron Deflector (U-238)
[9] - Conventional Explosive Charge(s)
[10] - Plutonium (Pu-239)
[11] - Receptacle for Beryllium/Polonium mixture to facilitate atomic detonation reaction.
[12] - Fuses (inserted to arm bomb)
II. Nuclear Fission/Nuclear Fusion
------------------------------
There are 2 types of atomic explosions that can be facilitated by U-
235; fission and fusion. Fission, simply put, is a nuclear reaction in which
an atomic nucleus splits into fragments, usually two fragments of comparable
mass, with the evolution of approximately 100 million to several hundred
million volts of energy. This energy is expelled explosively and
violently in the atomic bomb. A fusion reaction is invariably started with a fission
reaction, but unlike the fission reaction, the fusion (Hydrogen) bomb
derives its power from the fusing of nuclei of various hydrogen isotopes in the
formation of helium nuclei. Being that the bomb in this file is strictly
atomic, the other aspects of the Hydrogen Bomb will be set aside for now.
The massive power behind the reaction in an atomic bomb arises from
the forces that hold the atom together. These forces are akin to, but not
quite the same as, magnetism.
Atoms are comprised of three sub-atomic particles. Protons and neutrons
cluster together to form the nucleus (central mass) of the atom while the
electrons orbit the nucleus much like planets around a sun. It is these
particles that determine the stability of the atom.
Most natural elements have very stable atoms which are impossible to
split except by bombardment by particle accelerators. For all practical
purposes, the one true element whose atoms can be split comparatively
easily is the metal Uranium. Uranium's atoms are unusually large, henceforth,
it is hard for them to hold together firmly. This makes Uranium-235 an
exceptional candidate for nuclear fission.
Uranium is a heavy metal, heavier than gold, and not only does it
have the largest atoms of any natural element, the atoms that comprise Uranium
have far more neutrons than protons. This does not enhance their capacity to
split, but it does have an important bearing on their capacity to
facilitate an explosion.
There are two isotopes of Uranium. Natural Uranium consists mostly
of isotope U-238, which has 92 protons and 146 neutrons (92+146=238). Mixed
with this isotope, one will find a 0.6% accumulation of U-235, which has only
143 neutrons. This isotope, unlike U-238, has atoms that can be split, thus
it is termed "fissionable" and useful in making atomic bombs. Being that U-238
is neutron-heavy, it reflects neutrons, rather than absorbing them like its
brother isotope, U-235. (U-238 serves no function in an atomic reaction,
but its properties provide an excellent shield for the U-235 in a constructed
bomb as a neutron reflector. This helps prevent an accidental chain reaction
between the larger U-235 mass and its `bullet' counterpart within the
bomb.
Also note that while U-238 cannot facilitate a chain-reaction, it can be
neutron-saturated to produce Plutonium (Pu-239). Plutonium is
fissionable and can be used in place of Uranium-235 {albeit, with a different model of
detonator} in an atomic bomb. [See Sections 3 & 4 of this file.])
Both isotopes of Uranium are naturally radioactive. Their bulky
atoms disintegrate over a period of time. Given enough time, (over 100,000
years or more) Uranium will eventually lose so many particles that it will turn
into the metal lead. However, this process can be accelerated. This process
is known as the chain reaction. Instead of disintegrating slowly, the atoms
are forcibly split by neutrons forcing their way into the nucleus. A U-235
atom is so unstable that a blow from a single neutron is enough to split it
and henceforth bring on a chain reaction. This can happen even when a
critical mass is present. When this chain reaction occurs, the Uranium atom
splits into two smaller atoms of different elements, such as Barium and Krypton.
When a U-235 atom splits, it gives off energy in the form of heat and
Gamma radiation, which is the most powerful form of radioactivity and the
most lethal. When this reaction occurs, the split atom will also give off two
or three of its `spare' neutrons, which are not needed to make either Barium
or Krypton. These spare neutrons fly out with sufficient force to split
other atoms they come in contact with. [See chart below] In theory, it is
necessary to split only one U-235 atom, and the neutrons from this will
split other atoms, which will split more...so on and so forth. This
progression does not take place arithmetically, but geometrically. All of this will
happen within a millionth of a second.
The minimum amount to start a chain reaction as described above is
known as SuperCritical Mass. The actual mass needed to facilitate this chain
reaction depends upon the purity of the material, but for pure U-235, it
is 110 pounds (50 kilograms), but no Uranium is never quite pure, so in
reality more will be needed.
Uranium is not the only material used for making atomic bombs.
Another material is the element Plutonium, in its isotope Pu-239. Plutonium is
not found naturally (except in minute traces) and is always made from
Uranium. The only way to produce Plutonium from Uranium is to process U-238
through a nuclear reactor. After a period of time, the intense radioactivity
causes the metal to pick up extra particles, so that more and more of its atoms turn
into Plutonium.
Plutonium will not start a fast chain reaction by itself, but this
difficulty is overcome by having a neutron source, a highly radioactive
material that gives off neutrons faster than the Plutonium itself. In
certain types of bombs, a mixture of the elements Beryllium and Polonium is used
to bring about this reaction. Only a small piece is needed. The material
is not fissionable in and of itself, but merely acts as a catalyst to the
greater reaction.
=========================================================================
---------------------- Diagram Outline -
---------------------
[1] - Incoming Neutron
[2] - Uranium-235
[3] - Uranium-236
[4] - Barium Atom
[5] - Krypton Atom
=========================================================================
-End of section 2-
-Diagrams & Documentation of the Atomic Bomb-
--------------------------------
File courtesy of Outlaw Labs
=========================================================================
--------------------------------
III. The Mechanism of The Bomb-------------------------
Altimeter
---------
An ordinary aircraft altimeter uses a type of Aneroid Barometer
which measures the changes in air pressure at different heights. However,
changes in air pressure due to the weather can adversely affect the altimeter's
readings. It is far more favorable to use a radar (or radio) altimeter
for enhanced accuracy when the bomb reaches Ground Zero.
While Frequency Modulated-Continuous Wave (FM CW) is more
complicated, the accuracy of it far surpasses any other type of altimeter. Like
simple pulse systems, signals are emitted from a radar aerial (the bomb),
bounced off the ground and received back at the bomb's altimeter. This pulse system
applies to the more advanced altimeter system, only the signal is
continuous and centered around a high frequency such as 4200 MHz. This signal is
arranged to steadily increase at 200 MHz per interval before dropping
back to its original frequency.
As the descent of the bomb begins, the altimeter transmitter will send
out a pulse starting at 4200 MHz. By the time that pulse has returned, the
altimeter transmitter will be emitting a higher frequency. The
difference depends on how long the pulse has taken to do the return journey. When
these two frequencies are mixed electronically, a new frequency (the difference
between the two) emerges. The value of this new frequency is measured by
the built-in microchips. This value is directly proportional to the distance
travelled by the original pulse, so it can be used to give the actual
height.
In practice, a typical FM CW radar today would sweep 120 times per
second. Its range would be up to 10,000 feet (3000 m) over land and
20,000 feet (6000 m) over sea, since sound reflections from water surfaces are
clearer.
The accuracy of these altimeters is within 5 feet (1.5 m) for the higher
ranges. Being that the ideal airburst for the atomic bomb is usually set
for 1,980 feet, this error factor is not of enormous concern.
The high cost of these radar-type altimeters has prevented their use
in commercial applications, but the decreasing cost of electronic components
should make them competitive with barometric types before too long.
Air Pressure Detonator
----------------------
The air pressure detonator can be a very complex mechanism, but for
all practical purposes, a simpler model can be used. At high altitudes, the
air is of lesser pressure. As the altitude drops, the air pressure
increases. A simple piece of very thin magnetized metal can be used as an air pressure
detonator. All that is needed is for the strip of metal to have a bubble
of extremely thin metal forged in the center and have it placed directly
underneath the electrical contact which will trigger the conventional
explosive detonation. Before setting the strip in place, push the bubble in
so that it will be inverted.
Once the air pressure has achieved the desired level, the magnetic
bubble will snap back into its original position and strike the contact, thus
completing the circuit and setting off the explosive(s).
Detonating Head
---------------
The detonating head (or heads, depending on whether a Uranium or
Plutonium bomb is being used as a model) that is seated in the
conventional explosive charge(s) is similar to the standard-issue blasting cap. It
merely serves as a catalyst to bring about a greater explosion. Calibration of
this device is essential. Too small of a detonating head will only cause a
colossal dud that will be doubly dangerous since someone's got to disarm
and re-fit the bomb with another detonating head. (an added measure of
discomfort comes from the knowledge that the conventional explosive may have
detonated with insufficient force to weld the radioactive metals. This will cause
a supercritical mass that could go off at any time.) The detonating head
will receive an electric charge from the either the air pressure detonator or
the radar altimeter's coordinating detonator, depending on what type of
system is used. The Du Pont company makes rather excellent blasting caps that can
be easily modified to suit the required specifications.
Conventional Explosive Charge(s)
--------------------------------
This explosive is used to introduce (and weld) the lesser amount of
Uranium to the greater amount within the bomb's housing. [The amount of
pressure needed to bring this about is unknown and possibly classified by
the United States Government for reasons of National Security]
Plastic explosives work best in this situation since they can be
manipulated to enable both a Uranium bomb and a Plutonium bomb to
detonate.
One very good explosive is Urea Nitrate. The directions on how to make
Urea
Nitrate are as follows:
- Ingredients -
---------------
[1] 1 cup concentrated solution of uric acid (C5 H4 N4 O3)
[2] 1/3 cup of nitric acid
[3] 4 heat-resistant glass containers
[4] 4 filters (coffee filters will do)
Filter the concentrated solution of uric acid through a filter to
remove
impurities. Slowly add 1/3 cup of nitric acid to the solution and let
the mixture stand for 1 hour. Filter again as before. This time the Urea
Nitrate crystals will collect on the filter. Wash the crystals by pouring water
over them while they are in the filter. Remove the crystals from the filter
and allow 16 hours for them to dry. This explosive will need a blasting cap
to detonate.
It may be necessary to make a quantity larger than the
aforementioned list calls for to bring about an explosion great enough to cause the
Uranium (or Plutonium) sections to weld together on impact.
Neutron Deflector
-----------------
The neutron deflector is comprised solely of Uranium-238. Not only
is U-238 non-fissionable, it also has the unique ability to reflect neutrons
back to their source.
The U-238 neutron deflector can serve 2 purposes. In a Uranium
bomb, the neutron deflector serves as a safeguard to keep an accidental
supercritical mass from occurring by bouncing the stray neutrons from the `bullet'
counterpart of the Uranium mass away from the greater mass below it (and
viceversa).
The neutron deflector in a Plutonium bomb actually helps the
wedges of Plutonium retain their neutrons by `reflecting' the stray particles
back into the center of the assembly. [See diagram in Section 4 of this
file.]
Uranium & Plutonium
-------------------
Uranium-235 is very difficult to extract. In fact, for every 25,000
tons of Uranium ore that is mined from the earth, only 50 tons of Uranium
metal can be refined from that, and 99.3% of that metal is U-238 which is too
stable to be used as an active agent in an atomic detonation. To make matters even
more complicated, no ordinary chemical extraction can separate the two
isotopes since both U-235 and U-238 possess precisely identical chemical
characteristics. The only methods that can effectively separate U-235 from
U-238 are mechanical methods.
U-235 is slightly, but only slightly, lighter than its counterpart,
U-238. A system of gaseous diffusion is used to begin the separating
process between the two isotopes. In this system, Uranium is combined with
fluorine to form Uranium Hexafluoride gas. This mixture is then propelled by lowpressure
pumps through a series of extremely fine porous barriers.
Because
the U-235 atoms are lighter and thus propelled faster than the U-238
atoms, they could penetrate the barriers more rapidly. As a result, the
U-235's concentration became successively greater as it passed through
each barrier. After passing through several thousand barriers, the Uranium
Hexafluoride contains a relatively high concentration of U-235 -- 2% pure
Uranium in the case of reactor fuel, and if pushed further could
(theoretically) yield up to 95% pure Uranium for use in an atomic bomb.
Once the process of gaseous diffusion is finished, the Uranium must
be refined once again. Magnetic separation of the extract from the previous
enriching process is then implemented to further refine the Uranium.
This involves electrically charging Uranium Tetrachloride gas and directing it
past a weak electromagnet. Since the lighter U-235 particles in the gas
stream are less affected by the magnetic pull, they can be gradually separated from
the flow.
Following the first two procedures, a third enrichment process is
then applied to the extract from the second process. In this procedure, a gas
centrifuge is brought into action to further separate the lighter U-235
from its heavier counter-isotope. Centrifugal force separates the two
isotopes of Uranium by their mass. Once all of these procedures have been completed,
all that need be done is to place the properly molded components of Uranium-
235 inside a warhead that will facilitate an atomic detonation.
Supercritical mass for Uranium-235 is defined as 110 lbs (50 kgs) of
pure Uranium.
Depending on the refining process(es) used when purifying the U-235
for use, along with the design of the warhead mechanism and the altitude at
which it detonates, the explosive force of the A-bomb can range anywhere from 1
kiloton (which equals 1,000 tons of TNT) to 20 megatons (which equals 20
million tons of TNT -- which, by the way, is the smallest strategic
nuclear warhead we possess today. {Point in fact -- One Trident Nuclear
Submarine carries as much destructive power as 25 World War II's}).
While Uranium is an ideally fissionable material, it is not the only
one.
Plutonium can be used in an atomic bomb as well. By leaving U-238 inside
an atomic reactor for an extended period of time, the U-238 picks up extra
particles (neutrons especially) and gradually is transformed into the
element Plutonium.
Plutonium is fissionable, but not as easily fissionable as Uranium.
While Uranium can be detonated by a simple 2-part gun-type device,
Plutonium must be detonated by a more complex 32-part implosion chamber along with
a stronger conventional explosive, a greater striking velocity and a
simultaneous triggering mechanism for the conventional explosive packs.
Along with all of these requirements comes the additional task of introducing a
fine mixture of Beryllium and Polonium to this metal while all of these
actions are occurring.
Supercritical mass for Plutonium is defined as 35.2 lbs (16 kgs).
This amount needed for a supercritical mass can be reduced to a smaller
quantity of 22 lbs (10 kgs) by surrounding the Plutonium with a U-238 casing.
To illustrate the vast difference between a Uranium gun-type
detonator and a Plutonium implosion detonator, here is a quick rundown.
=========================================================================
[1] Uranium Detonator
-----------------
Comprised of 2 parts. Larger mass is spherical and
concave.
Smaller mass is precisely the size and shape of the
`missing' section of the larger mass. Upon detonation of
conventional explosive, the smaller mass is violently injected and
welded to the larger mass. Supercritical mass is reached, chain
reaction follows in one millionth of a second.
[2] Plutonium Detonator
-------------------
Comprised of 32 individual 45-degree pie-shaped sections of
Plutonium surrounding a Beryllium/Polonium mixture. These
32 sections together form a sphere. All of these sections
must have the precisely equal mass (and shape) of the others.
The shape of the detonator resembles a soccerball. Upon
detonation of conventional explosives, all 32 sections must merge with
the B/P mixture within 1 ten-millionths of a second.
=========================================================================
Lead Shield
-----------
The lead shield's only purpose is to prevent the inherent
radioactivity of the bomb's payload from interfering with the other mechanisms of the
bomb.
The neutron flux of the bomb's payload is strong enough to short circuit
the internal circuitry and cause an accidental or premature detonation.
Fuses
-----
The fuses are implemented as another safeguard to prevent an
accidental detonation of both the conventional explosives and the nuclear payload.
These fuses are set near the surface of the `nose' of the bomb so that they can
be installed easily when the bomb is ready to be launched. The fuses should
be installed only shortly before the bomb is launched. To affix them before
it is time could result in an accident of catastrophic proportions.
=========================================================================
-End of section 3-
-Documentation & Diagrams of the Atomic Bomb-
--------------------------------
File courtesy of Outlaw Labs
--------------------------------
=========================================================================
IV. The Diagram of the Atomic Bomb
------------------------------
[Gravity Bomb Model]
----------------------------
------------------------------
[Gravity Bomb Model]
----------------------------
=========================================================================
---------------------- Diagram Outline -
---------------------
[1] - Tail Cone
[2] - Stabilizing Tail Fins
[3] - Air Pressure Detonator
[4] - Air Inlet Tube(s)
[5] - Altimeter/Pressure Sensors
[6] - Lead Shield Container
[7] - Detonating Head
[8] - Conventional Explosive Charge
[9] - Packing
[10] - Uranium (U-235) [Plutonium (See other diagram)]
[11] - Neutron Deflector (U-238)
[12] - Telemetry Monitoring Probes
[13] - Receptacle for U-235 upon detonation
to facilitate supercritical mass.
[14] - Fuses (inserted to arm bomb)
---------------------
- Diagram Outline ----------------------
[1] - Tail Cone
[2] - Stabilizing Tail Fins
[3] - Air Pressure Detonator
[4] - Air Inlet Tube(s)
[5] - Altimeter/Pressure Sensors
[6] - Electronic Conduits & Fusing Circuits
[7] - Lead Shield Container
[8] - Neutron Deflector (U-238)
[9] - Conventional Explosive Charge(s)
[10] - Plutonium (Pu-239)
[11] - Receptacle for Beryllium/Polonium mixture to facilitate atomic detonation reaction.
[12] - Fuses (inserted to arm bomb)
========================================================================= ========================================================================= =========================================================================
É isso, divirtam-se, crianças, compartilhem o blog com seus amigos, e, caso queiram algum tutorial específico, deixem nos comentários e ele pode ser atendido [como esse foi].











Entendi tudo,obrigado pelo tutorial,tentarei aqui em casa iraiariariar
ResponderExcluir